Keep the Beer Cold!
I was thinking about building a super-insulated icebox, and letting the drip-water drain into a pan under the icebox. This icebox will be for my dry food (cheese, lettuce, fruit, eggs, etc) that I don’t want waterlogged.  Anyway, in that drip pan I was going to store some 12-oz cans and cool them with the drip-water. The lift-off top is for adding an ice block. I was going to have a front door to gain access to my cheesy-cheese and beer.
Anyway, in that drip pan I was going to store some 12-oz cans and cool them with the drip-water. The lift-off top is for adding an ice block. I was going to have a front door to gain access to my cheesy-cheese and beer.
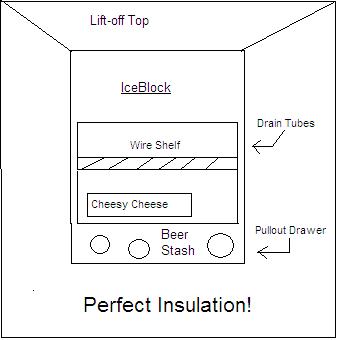
Hillbilly engineering at its best!
1 BTU (British Thermal Unit) is the amount of heat that is required to raise the temperature of 1 pound of water through 1 degree Fahrenheit. This is heat added, but it works both ways: 1 BTU of heat needs to be removed to lower 1 pound of water through 1 degree Fahrenheit. This is also the definition for the Specific Heat of Water, which = 1 (no units).
So what? Well…. one gallon of water weighs 8.333 pounds at 65 degrees F. At 8 pints to a gallon, one pint (16 fluid ounces) weighs 1.04125 pounds. A 12-oz drink weighs 1.04125 x 0.75 = 0.78 pounds. The 0.75 is three-quarters of 16 fluid ounces, or 12 fluid ounces.
Well…. one gallon of water weighs 8.333 pounds at 65 degrees F. At 8 pints to a gallon, one pint (16 fluid ounces) weighs 1.04125 pounds. A 12-oz drink weighs 1.04125 x 0.75 = 0.78 pounds. The 0.75 is three-quarters of 16 fluid ounces, or 12 fluid ounces.
In this case, 16 fluid ounces does NOT equal a pound, but it’s close. It’s easy to get confused between fluid ounces and Avoirdupois ounces (where 16 ounces really equals a pound). Might be why most of the rest of the world uses the metric system.
Might be why most of the rest of the world uses the metric system.  Or they’re not up to the challenge.
Or they’re not up to the challenge. 

In any event, it takes a 12-oz beverage (at 70 degrees F) 38 x 0.78 BTU removed to cool to 32 degrees F. Or 29.64 BTUs total removal. The 38 represents going from 70 to 32 degrees. The 0.78 is the BTU removal requirement (per pound) of the 12-oz drink.
A six-pack needs 177.84 BTU’s removed (6 x 29.64 BTU per can). Ya gotta love the Brits for that unit. If you’re handed a 12-oz beverage that’s at 32 degrees F, you’re gittin’ a cold one!

Heat of fusion: The number of calories of heat energy required to melt one gram of any substance without producing any change in its temperature is called the heat of fusion of that substance. Well…. yeah!… and how can something be heated without raising its temperature?
 Voodoo science?
Voodoo science?  Nope; by melting ice at 32 degrees that’s floating in water at 32 degrees; the water temperature won’t go up until the ice has completely melted.
Nope; by melting ice at 32 degrees that’s floating in water at 32 degrees; the water temperature won’t go up until the ice has completely melted. 
The heat of fusion of ICE is 80. It takes 80 calories to melt one gram of 0-degree Celsius Ice into 0-degree Celsius water. Reverse: It takes 80 calories to freeze 1 gram of 0-degree Celsius water into 0-degree Celsius ice. That’s 80 times the water’s specific heat. Yawn…
O.K. Back to BTU’s
Since 252 Calories = 1 BTU, 1 gram of ice needs 80 calories/252 BTU’s per calorie to melt; the calories drop out of the equation, leaving 0.3175 BTU’s. One pound [Avoirdupois, where 1 lb =16 oz.] equals 453.59 grams. So 1 pound of 32-degree F ice needs 144 BTU’s added to melt into 32-degree F water. (0.3175 BTU’s x 453.59 grams per pound)
My calculator’s gettin’ hot. Now taking the 8.333 pounds for one gallon of frozen water, it requires 1,199.52 BTU’s added to melt that gallon of ice into one gallon of water at 32-degrees F. (144 BTU’s per pound x 8.333 pounds) This is the heat of fusion at work. Once the ice has completely melted, I still have 8.333 pounds of 32-degree water with specific heat remaining.
Now taking the 8.333 pounds for one gallon of frozen water, it requires 1,199.52 BTU’s added to melt that gallon of ice into one gallon of water at 32-degrees F. (144 BTU’s per pound x 8.333 pounds) This is the heat of fusion at work. Once the ice has completely melted, I still have 8.333 pounds of 32-degree water with specific heat remaining.
Relentlessly continuing the math: 1,999.52 BTU’s divided by 29.64 BTU’s per 12-oz bev equals 67.46 cans of beer cooled. The specific heat of the remaining 32-deg F melt-water is 8.333 pounds x 1 BTU per pound = 8.333 BTU.
Hmmm… Can I use that remaining specific heat to cool another 0.56 can of Beer, making an even 68 cans cool? I’m so frugal.
Can I use that remaining specific heat to cool another 0.56 can of Beer, making an even 68 cans cool? I’m so frugal.  The math says 0.56 (56%) remaining liquid x 0.78 pounds (remember, one 12-oz drink = 0.78 lbs) equal 0.4368 BTU per-degree F the can needs to lose. I bang my calculator to find 8.333 BTU specific heat available divided by 0.4368 BTU per-degree drop equals (BTU’s cancel out of the equation) 19.07 degrees drop. The 0.56 can of liquid started at 70 degrees F, so it will only drop 70-19.07 degrees F, or down to 50.93 degrees. Yikes!
The math says 0.56 (56%) remaining liquid x 0.78 pounds (remember, one 12-oz drink = 0.78 lbs) equal 0.4368 BTU per-degree F the can needs to lose. I bang my calculator to find 8.333 BTU specific heat available divided by 0.4368 BTU per-degree drop equals (BTU’s cancel out of the equation) 19.07 degrees drop. The 0.56 can of liquid started at 70 degrees F, so it will only drop 70-19.07 degrees F, or down to 50.93 degrees. Yikes!  Not for me.
Not for me.  Dump it on the hot brats and kraut.
Dump it on the hot brats and kraut. 
Darn. . It would take the specific heat in at least 4 gallons of melt-water to properly cool one can. I also ignored the can heat itself, and the heat of sugar and other non-water contents of the can. As you might have noticed, no time was involved with cooling the beer, just BTU transfer.
. It would take the specific heat in at least 4 gallons of melt-water to properly cool one can. I also ignored the can heat itself, and the heat of sugar and other non-water contents of the can. As you might have noticed, no time was involved with cooling the beer, just BTU transfer.  Even so, I find the drip-water drain pan not worth the space or time to build, and that’s with perfect insulation (no heat loss or gain). As soon as I find that perfect insulation I will also be able to cool 67 full cans of beer with one gallon of ice. In the meantime, I’ll keep my beer submerged in the water of melting ice inside a good cooler. Beer-can marinade, so to speak.
Even so, I find the drip-water drain pan not worth the space or time to build, and that’s with perfect insulation (no heat loss or gain). As soon as I find that perfect insulation I will also be able to cool 67 full cans of beer with one gallon of ice. In the meantime, I’ll keep my beer submerged in the water of melting ice inside a good cooler. Beer-can marinade, so to speak. 

What else have I learned?
1. Keep the melt-water (Specific heat) with the ice cubes (Heat of fusion) together at all times. It keeps the whole combination at 32 F until the ice is gone.
2. One six-pack needs to lose about 180 BTU to cool from 70 to 32 F. (So don’t buy warm beer! Duh! Even Hillbillies know bettern’ that. )
3. One gallon of ice (Milk Jug) should cool about 1,200 BTU.
4. One pound of ice has about a 145 total BTU cooling capacity.
5. The Specific Heat of Ice (below 32 F) = 0.504. 1 degree F rise = 0.504 BTU per pound. Not much heat suckin’ ability there. Specific Heat of Water = 1.
6. When all the BTU’s are gone from the ice, you need to drink the beer fast; it’s getting warm!! The ice should really outlast the beer in a perfect world.

7. Insulation is worth its weight in gold.
8. Math might save time and money. Sometimes. Maybe?
9. Cold Beer! Ya!….das iz goot bier!
Cheers!
Hillbilly Gene
 Anyway, in that drip pan I was going to store some 12-oz cans and cool them with the drip-water. The lift-off top is for adding an ice block. I was going to have a front door to gain access to my cheesy-cheese and beer.
Anyway, in that drip pan I was going to store some 12-oz cans and cool them with the drip-water. The lift-off top is for adding an ice block. I was going to have a front door to gain access to my cheesy-cheese and beer.
Hillbilly engineering at its best!
1 BTU (British Thermal Unit) is the amount of heat that is required to raise the temperature of 1 pound of water through 1 degree Fahrenheit. This is heat added, but it works both ways: 1 BTU of heat needs to be removed to lower 1 pound of water through 1 degree Fahrenheit. This is also the definition for the Specific Heat of Water, which = 1 (no units).
So what?
 Well…. one gallon of water weighs 8.333 pounds at 65 degrees F. At 8 pints to a gallon, one pint (16 fluid ounces) weighs 1.04125 pounds. A 12-oz drink weighs 1.04125 x 0.75 = 0.78 pounds. The 0.75 is three-quarters of 16 fluid ounces, or 12 fluid ounces.
Well…. one gallon of water weighs 8.333 pounds at 65 degrees F. At 8 pints to a gallon, one pint (16 fluid ounces) weighs 1.04125 pounds. A 12-oz drink weighs 1.04125 x 0.75 = 0.78 pounds. The 0.75 is three-quarters of 16 fluid ounces, or 12 fluid ounces.
In this case, 16 fluid ounces does NOT equal a pound, but it’s close. It’s easy to get confused between fluid ounces and Avoirdupois ounces (where 16 ounces really equals a pound).
 Might be why most of the rest of the world uses the metric system.
Might be why most of the rest of the world uses the metric system.  Or they’re not up to the challenge.
Or they’re not up to the challenge. 

In any event, it takes a 12-oz beverage (at 70 degrees F) 38 x 0.78 BTU removed to cool to 32 degrees F. Or 29.64 BTUs total removal. The 38 represents going from 70 to 32 degrees. The 0.78 is the BTU removal requirement (per pound) of the 12-oz drink.
A six-pack needs 177.84 BTU’s removed (6 x 29.64 BTU per can). Ya gotta love the Brits for that unit. If you’re handed a 12-oz beverage that’s at 32 degrees F, you’re gittin’ a cold one!


Heat of fusion: The number of calories of heat energy required to melt one gram of any substance without producing any change in its temperature is called the heat of fusion of that substance. Well…. yeah!… and how can something be heated without raising its temperature?

 Voodoo science?
Voodoo science?  Nope; by melting ice at 32 degrees that’s floating in water at 32 degrees; the water temperature won’t go up until the ice has completely melted.
Nope; by melting ice at 32 degrees that’s floating in water at 32 degrees; the water temperature won’t go up until the ice has completely melted. 
The heat of fusion of ICE is 80. It takes 80 calories to melt one gram of 0-degree Celsius Ice into 0-degree Celsius water. Reverse: It takes 80 calories to freeze 1 gram of 0-degree Celsius water into 0-degree Celsius ice. That’s 80 times the water’s specific heat. Yawn…

O.K. Back to BTU’s

Since 252 Calories = 1 BTU, 1 gram of ice needs 80 calories/252 BTU’s per calorie to melt; the calories drop out of the equation, leaving 0.3175 BTU’s. One pound [Avoirdupois, where 1 lb =16 oz.] equals 453.59 grams. So 1 pound of 32-degree F ice needs 144 BTU’s added to melt into 32-degree F water. (0.3175 BTU’s x 453.59 grams per pound)
My calculator’s gettin’ hot.
 Now taking the 8.333 pounds for one gallon of frozen water, it requires 1,199.52 BTU’s added to melt that gallon of ice into one gallon of water at 32-degrees F. (144 BTU’s per pound x 8.333 pounds) This is the heat of fusion at work. Once the ice has completely melted, I still have 8.333 pounds of 32-degree water with specific heat remaining.
Now taking the 8.333 pounds for one gallon of frozen water, it requires 1,199.52 BTU’s added to melt that gallon of ice into one gallon of water at 32-degrees F. (144 BTU’s per pound x 8.333 pounds) This is the heat of fusion at work. Once the ice has completely melted, I still have 8.333 pounds of 32-degree water with specific heat remaining.
Relentlessly continuing the math: 1,999.52 BTU’s divided by 29.64 BTU’s per 12-oz bev equals 67.46 cans of beer cooled. The specific heat of the remaining 32-deg F melt-water is 8.333 pounds x 1 BTU per pound = 8.333 BTU.
Hmmm…
 Can I use that remaining specific heat to cool another 0.56 can of Beer, making an even 68 cans cool? I’m so frugal.
Can I use that remaining specific heat to cool another 0.56 can of Beer, making an even 68 cans cool? I’m so frugal.  The math says 0.56 (56%) remaining liquid x 0.78 pounds (remember, one 12-oz drink = 0.78 lbs) equal 0.4368 BTU per-degree F the can needs to lose. I bang my calculator to find 8.333 BTU specific heat available divided by 0.4368 BTU per-degree drop equals (BTU’s cancel out of the equation) 19.07 degrees drop. The 0.56 can of liquid started at 70 degrees F, so it will only drop 70-19.07 degrees F, or down to 50.93 degrees. Yikes!
The math says 0.56 (56%) remaining liquid x 0.78 pounds (remember, one 12-oz drink = 0.78 lbs) equal 0.4368 BTU per-degree F the can needs to lose. I bang my calculator to find 8.333 BTU specific heat available divided by 0.4368 BTU per-degree drop equals (BTU’s cancel out of the equation) 19.07 degrees drop. The 0.56 can of liquid started at 70 degrees F, so it will only drop 70-19.07 degrees F, or down to 50.93 degrees. Yikes!  Not for me.
Not for me.  Dump it on the hot brats and kraut.
Dump it on the hot brats and kraut. 
Darn.
 . It would take the specific heat in at least 4 gallons of melt-water to properly cool one can. I also ignored the can heat itself, and the heat of sugar and other non-water contents of the can. As you might have noticed, no time was involved with cooling the beer, just BTU transfer.
. It would take the specific heat in at least 4 gallons of melt-water to properly cool one can. I also ignored the can heat itself, and the heat of sugar and other non-water contents of the can. As you might have noticed, no time was involved with cooling the beer, just BTU transfer.  Even so, I find the drip-water drain pan not worth the space or time to build, and that’s with perfect insulation (no heat loss or gain). As soon as I find that perfect insulation I will also be able to cool 67 full cans of beer with one gallon of ice. In the meantime, I’ll keep my beer submerged in the water of melting ice inside a good cooler. Beer-can marinade, so to speak.
Even so, I find the drip-water drain pan not worth the space or time to build, and that’s with perfect insulation (no heat loss or gain). As soon as I find that perfect insulation I will also be able to cool 67 full cans of beer with one gallon of ice. In the meantime, I’ll keep my beer submerged in the water of melting ice inside a good cooler. Beer-can marinade, so to speak. 

What else have I learned?

1. Keep the melt-water (Specific heat) with the ice cubes (Heat of fusion) together at all times. It keeps the whole combination at 32 F until the ice is gone.
2. One six-pack needs to lose about 180 BTU to cool from 70 to 32 F. (So don’t buy warm beer! Duh! Even Hillbillies know bettern’ that. )

3. One gallon of ice (Milk Jug) should cool about 1,200 BTU.
4. One pound of ice has about a 145 total BTU cooling capacity.
5. The Specific Heat of Ice (below 32 F) = 0.504. 1 degree F rise = 0.504 BTU per pound. Not much heat suckin’ ability there. Specific Heat of Water = 1.
6. When all the BTU’s are gone from the ice, you need to drink the beer fast; it’s getting warm!! The ice should really outlast the beer in a perfect world.


7. Insulation is worth its weight in gold.
8. Math might save time and money. Sometimes. Maybe?

9. Cold Beer! Ya!….das iz goot bier!

Cheers!
Hillbilly Gene

 Or pop
Or pop I’m designing my dry foodstuff icebox and wanted to use the drip-water to pre-cool a few beers. The saltwater trick will work in my dedicated beer cooler. The first couple of sips may have slightly salty margarita overtones, but I’ll just stick out my pinky and pretend the beer was a good year.
I’m designing my dry foodstuff icebox and wanted to use the drip-water to pre-cool a few beers. The saltwater trick will work in my dedicated beer cooler. The first couple of sips may have slightly salty margarita overtones, but I’ll just stick out my pinky and pretend the beer was a good year. 

